Thursday, September 13, 2012
About that paper on organic food...
Monday, August 22, 2011
Nature vs. Nurture in the gut microbiota III: what's in a name?
The population of microbes in our gut are a personal trait, like height or weight. The precise make-up of that population, its balance of different types of microbes, is significant as it can have major beneficial or harmful effects on our health. Like many other traits, the composition of that population is partly due to nature—our genes create an environment welcoming to certain types of microbes—and nurture, the food we eat.
Given the importance of the composition of the microbial community to our health, it’s not surprising that there is a lot of interest in trying to figure out just how significant are the contributions of nature and nurture. Comparing genetically identical mice with a drastically simplified community of microbes in their gut, researchers found that changes in diet led to predictable changes in community structure. However, comparing genetically distinct strains of laboratory mice, a different group of researchers found that the subtle genetic differences between two inbred lines of mice led to measurable differences in the gut microbial community (see part I).
The roles of nature and nurture can be probed further by comparing the microbial community of different, but related, types of animals. What if two animals are closely related, but have different diets? What if they are only distant relatives, but have similar diets? An extreme example of the latter case is a comparison of the intestinal microbes of the tammar wallaby and the cow (see part II). While the comparison is informative, these two mammals are about as distantly related as possible, so a closer comparison is useful.
In a recent study from Washington University at Saint Louis, a group led by Jeffrey Gordon attempted to compare a family tree of mammals, a family tree of the microbes that lived in their guts, the diets of those mammals, and the genetic abilities of their microbes. Any four-way comparison gets complicated pretty quickly, but there are some clear facts that emerge from the fog of statistics. These facts emphasize some basic biology of microbes, as well as telling us something about how mammals and their microbes co-evolve.
A family tree of the mammals comes out pretty much the same, regardless of whether you use genetic data or anatomical differences:
Looking at this family tree, there is little convergence between relatedness and diet. For instance, elephants, capybaras, gorillas, horses, and rabbits are all “hindgut-fermenting herbivores,” with a specific method of digesting cellulose—and yet, are found on different branches of the tree. Sheep, hyraxes, kangaroos, colobus monkeys, and pigs are all “foregut-fermenting herbivores,” with an anatomically distinct way of digesting plant matter—but again, they are only distant relatives. One can find groups, such as the primates, comprising foregut-fermenters, hindgut-fermenters, carnivores, and omnivores. So, genetics on a deep level does not determine diet.
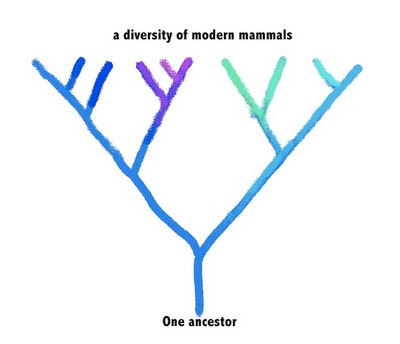
If genetic heritage doesn’t determine diet, does it at least determine something about a mammal’s intestinal microbial community? To approach this question, you can imagine a goodly branch of that mammalian family tree. A diverse bunch of modern mammals evolved from a common ancestor:
We obviously don’t know anything of the DNA sequence of their common ancestor, but we can see the genetic similarities of the modern animals, and work backwards to predict what their ancestor was like, as well as which modern animals are most closely related. If the ancestor had a certain genetic composition—symbolized by the color blue—then its descendants would have evolved variations on that genetic composition—symboized by variations in the hue in the modern beasts.
Now, if genetic heritage determines an animal’s gut microbes, then the genetic character of that community should show a pattern of evolution sort of like the mammalian family tree:
This is a trickier tree than the previous one. Rather than looking at a lineage of organisms, we are looking at the composition of a community of thousands of species. We can make an analogy with the white pages of a phone book: in this tree, we are looking at how the content of a community’s phone book changes over time. (Also, it’s important to remind ourselves that we don’t know what the original community was like—we only have the present one, and we try to work backwards.)
So, what did Gordon’s group find? If mammals and their intestinal microbes co-evolved, then we could examine a diversity of modern mammals and the microbial communities in their poop—which is just what they did. We’d expect related mammals to have related communities. Lining up the tips of the family trees (that is, just currently living animals and the microbes in their poop), we’d see something like this:
There was good reason to expect this result, as similar patterns of co-evolution are seen with insects and their symbiotic bacteria. Co-evolution is common. But here’s what they found:
What to make of this? Obviously, genetic heritage has very little to do with the community structure of an animal’s intestinal microbes. However, Gordon’s group looked at the same data again. This time, instead of looking at mammals by their ancestry, they considered their diet:
Aaaaah, much better! So, it seems like diet—nurture, in our nature/nurture dichotomy—is a much better predictor of a mammal’s microbial community. (This is a much simplified version of the data presented; my apologies to Gordon et al, who studied 33 mammal species and hundreds of microbial species. It’s also useful to note that in several instances they looked at the communities of different individuals of the same species, and found little variation between them.)
Interestingly, despite the differences in community structure between mammals, all mammals have pretty much the same “core” community of microbes. To return to the metaphor of a phone book, any big city in this country will have roughly the same collection of names, it’s just that one city will have a lot more Schmidts and Webers and Mullers and another will have many more Vasquezes and Diazes and Gonzaleses.
Although there was a decent correlation between mammalian diet and community composition in Gordon’s data, his group found an even more significant relationship. The best correlation was not between mammalian diet and community composition. It was between mammalian diet and what genes were present in the microbial community.
Let’s consider that phone book analogy again. If I wanted to find somebody to turn some wheat into flour, I probably wouldn’t turn to the white pages and call up somebody named Muller or Miller. (Heck, I wouldn’t say I’m a good horticulturalist because I’m named Appleman.) Trades are not inherited with family names, and this is true in the microbial world as well. So, if you’re a vegetarian mammal looking for a microbe to turn your b-glucosides into monosaccharides, you can’t assume that you’re looking for Brevibacter—all you care about is whether the organism has the genes for doing the job.
Microbial genomes—the collection of genes a given species has—are incredibly fluid. Genes can disappear from one genome in the space of a few generations, and can establish themselves in a new genome in an equally short time. Genes can travel with relative ease between bacterial species that are barely related to each other. (There are many mechanisms for doing this, and it makes firmly describing a microbial species next to impossible.) So, when a hyrax feeds its intestinal microbes a blend of grass and insects, it is (unwittingly) selecting a specific assemblage of genes, not a specific community of microbes.
These results raise some interesting questions about evolution. The community of microbes that lives in a mammal’s gut—its microbiome—can easily be viewed as an organ like the lungs or kidneys. However, its inheritance and evolution are quite distinct from that of the rest of the animal. As Gordon’s group suggests, it would be fun to compare a lot of different carnivores, and try to extrapolate just when differences in their microbiomes evolved. Do such evolutionary jumps correlate with events such as continental drift or other significant milestones? Are the changes gradual or rapid? Can one community be dislocated by another? Just how fluid are the communities in the gut?
These questions bring us back to the tammar wallaby and its oddly non-methanogenic digestion. Interestingly, in this study, the microbiome of a kangaroo clearly grouped with other foregut-fermenting mammals, such as sheep and gazelles. It was an outlier in that group, but still clearly part of it and not some other group. Well, the tammar wallaby was found to have a small number of methanogens and acetogens in its gut, giving it at least some similarity to the sheep. The big difference in digestion (and flatus) may be traceable to the very small community difference of one organism with one interesting metabolic pathway, a factor that simply can’t be revealed by this sort of analysis. The situation is reminiscent of human societies, which can be hugely affected by a single individual—and knowing the name of that individual won’t tell you anything about how they affect society.
Brian D. Muegge, Justin Kucyznski, Dan Knigts, Jose C. Clemente, Antonio Gonzalez, Luigi Fontana, Bernard Henrissat, Rob Knight, and Jeffrey Gordon (2011). Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science 332, 970-974.
Wednesday, August 17, 2011
Nature vs Nurture in the gut microbiota, part II: the music of wallaby farts
I recently wrote about a study showing the impact of host genetics on gut microbes. This was done by comparing different strains of mice, eating about the same diet. These animals are closely related, having had common ancestors only a hundred years ago. What if we looked at animals eating about the same diet, but whose most recent common ancestor lived a hundred million years ago?
Consider the tammar wallaby: This cute little kangaroo-let has recently found interest as a laboratory animal. As a marsupial, the last ancestor it shares with a cow lived over a hundred million years ago. It eats essentially the same diet as a cow. However, while cow farts are rich in methane, wallaby farts are not. This suggests that, despite identical diets, these animals have very different gut microbes. Until very recently, the nature of these differences was quite puzzling.
It’s useful to have a little background on what goes on in the gut of a cow eating grass. Most of what the animal eats is cellulose and other polysaccharides. These are compounds that are made by stringing together hundreds of sugar molecules, and they are tough—the table that I’m writing on and the cotton I’m wearing are made of polysaccharides. Breaking these polysaccharides down to release their constituent sugars is chemically very difficult, and breaking down the cellulose found in forage is almost impossible. Surprisingly, cows can not do this. It’s a job done by microbes living in the guts of these animals. Of course, the microbes that release the sugars from cellulose don’t do it out of charity to their hosts—as soon as the sugar is freed from the cellulose, they eat it. How does this help the cow?
You probably know fermentation as what yeasts do, taking sugar and making ethanol and CO2 as wastes. However, there are other ways that microbes get energy by fermenting, and most of the microbes in a cow’s gut take sugar and make acetic acid, CO2, and hydrogen gas as wastes. So, after the microbes have broken down the cellulose and fermented the sugars, the cow thanks the microbes nicely, and absorbs the acetic acid as a nutrient. But the CO2 and hydrogen pose a real problem.
Picture a worker on an assembly line: he takes two widgets, screws them together, and passes them on to the next guy. He can keep working as long as the widgets keep coming down the assembly line and the next guy takes his finished product away. If the next guy falls asleep, the product will build up, and our worker will be unable to do his job. This is what could happen to the fermentation in a cow: CO2 and hydrogen are the products of fermentation, and if they build up, the fermenting microbes will stop (and the cow will starve). Fortunately for the cow and the microbes, there are other microbes in its gut, called methanogens, that get energy by “burning” the hydrogen—they oxidize it, using CO2 instead of oxygen, and produce methane. So, the production of methane in cow farts is absolutely necessary if the cow is to continue breaking down cellulose.
However, as was noted, wallaby farts don’t have so much methane, even though they eat the same amount of cellulose. How can wallabies and their microbes digest cellulose?
One possibility that seemed likely was that there were some other microbes in the wallaby gut that were able to eat CO2 and hydrogen. It was already known that termites (who also eat cellulose) house bacteria called “acetogens” that combine hydrogen and CO2 to make acetic acid rather than methane. So, it was thought that if an animal ate cellulose, it had to have either methanogens or acetogens in its gut to help the fermentation reactions along (and in fact, we have some of each in our guts—as evidenced by the flammability of farts—just not as many, since we don’t eat as many polysaccharides). When I first heard that wallaby farts were low in methane, I assumed that wallabies must be full of acetogens.
However, it’s always good to check your assumptions. An analysis of the wallaby gut community showed few methanogens, and somewhat more acetogens—but not enough to make fermentation (as it was understood) work. This was a real puzzle—without microbes to consume the CO2 and hydrogen from fermentation, how could the wallaby eat cellulose? The answer, according to an international group of researchers, may be that methanogens and acetogens are unnecessary for the fermentation that happens in a wallaby gut—their microbes use a unique fermentation reaction that doesn’t produce hydrogen, and consumes CO2.
The overall theme of fermentation by the cow’s microbes is to break sugars into smaller and smaller pieces—start with a molecule of six carbons, then go to two molecules of three carbons, then two molecules of two carbons and two molecules of CO2 and some hydrogen atoms.
The researchers observed that a significant component of the microbial population of a wallaby gut was similar to a previously identified bacterium called Succinovibrio, named after its unusual metabolism. Sugar fermentation in Succinovibrio starts the same way as in the cow; however, in the final stages, the fragments of sugar molecules are partially reassembled to make a four-carbon compound, succinate. This process (which also consumes CO2) provides the bacterium energy to live—and what’s more, the succinate gives the wallaby more energy than a similar amount of acetate gives to a cow.
The researchers showed very nicely that this sort of fermentation can go on in the wallaby’s gut, and that this could explain the way that wallabies are able to eat the same diet as a cow yet not produce so much methane. So, why do wallabies play host to Succinovibrio while cows play host to methanogens? It’s obviously not diet—the original studies showing differences in methane production were done with wallabies and cows on the same feed, and wallabies do host a few methanogens. There may be structural issues; cow anatomy is quite different from that of the macropodes. But most likely, as we have learned from studies on mice and humans, is that the genetic background of the host sets up a molecular environment that is particularly hospitable to specific microbes. The genomes of both the cow and the wallaby have been sequenced, so the information is there. We just have to do the hard work of understanding it.
There is a practical aspect to this question, beyond the flammability of wallaby farts. Methane is an extremely powerful greenhouse gas; it is over twenty times more efficient at retaining solar heat than CO2. The human fondness for cattle grazing has increased methane production, contributing to global climate change. There are researchers who entertain the pipedream of converting cattle to a wallaby metabolism—producing more meat per munch of hay, and producing less pollution to boot. A noble cause, but I’ll probably only be able to eat a burger from such an animal when I pull up to the drive-through in my flying car.
Paul N. Evans, Lyn A. Hinds, Lindsay I. Sly, Christopher S. McSweeney, Mark Morrison, and Andr ́e-Denis G. Wright (2010). Community Composition and Density of Methanogens in the Foregut of the Tammar Wallaby (Macropus eugenii). Applied and Environmental Microbiology 75: 2598-2602.
Emma J. Gagen, Stuart E. Denman, Jagadish Padmanabha, Someshwar Zadbuke, Rafat Al Jassim, Mark Morrison, and Christopher S. McSweeney (2010). Functional Gene Analysis Suggests Different Acetogen Populations in the Bovine Rumen and Tammar Wallaby Forestomach. Applied and Environmental Microbiology 76: 7785–7795.
P. B. Pope, S. E. Denman, M. Jones, S. G. Tringe, K. Barry, S. A. Malfatti, A. C. McHardy, J.-F. Cheng, P. Hugenholtz, C. S. McSweeney, and M. Morrison (2010). Adaptation to herbivory by the Tammar wallaby includes bacterial and glycoside hydrolase profiles different from other herbivores. Proceedings Natl Acad Sci USA 107:14793-14798.
P. B. Pope, W. Smith, S. E. Denman, S. G. Tringe, K. Barry, P. Hugenholtz, C. S. McSweeney, A. C. McHardy, M. Morrison (2011). Isolation of Succinivibrionaceae Implicated in Low Methane Emissions from Tammar Wallabies. Science 333: 646-648.
Monday, August 15, 2011
Nature vs. Nurture in the gut microbiota
Although everyone has more or less similar general types of organisms in their gut, the balance of types varies considerably. Comparing the intestinal microbiota of two individuals is similar to comparing the yellow pages of Los Angeles and Fairbanks—they’ll both have the same classifications, but one will have a lot more acting coaches and the other will have more hunting guides. While the origin of the differences in yellow pages is largely due to environment, it’s more difficult to pin down the origin of the differences in our gut microbiota. Does diet have an effect on community composition? Does your genetics determine what microbes live in your gut?
A group from Washington University in St. Louis looked at an extremely simplified system to examine the influence of diet on gut microbes. Rather than studying humans, they used “gnotobiotic” mice—these are animals delivered by C-section, and raised in an absolutely sterile environment. These animals are pretty sickly (since the microbes that normally aid in development and digestion are absent), but researchers can deliberately add specific, known species of microbes to their food. This way, they know exactly what microbes are in there (gnotobiotic = “known life”), and by comparison with germ-free animals, they can know the effect of specific types of microbes.
To simplify their experiments, the researchers infected these mice with a community of 10 different types of microbes, chosen to represent the most general characteristics of the microbes in the human gut: one good at digesting starches, one good at fermenting amino acids, etc. They then gave these mice extremely simplified diets: mostly corn oil, mostly casein, mostly sucrose, or mostly cornstarch. After two weeks, the researchers collected the mouse poop, and analyzed the composition of the microbial community therein. This was repeated, until each mouse had enjoyed each diet.
After they had amassed a lot of mouse poop, they were able to develop a pretty good model of how the structure of the microbial community in the mouse gut was shaped by the mouse’s food intake. The model is actually relatively simple, and quite predictive: such a percent of protein in the diet leads to such a percent of Clostridium cells, and so on. The researchers were able to test their predictions by feeding the mice slightly more complex food (baby food, actually—pureed turkey, pureed carrots, etc). Sure enough, by knowing what went into the mouse, they were able to roughly predict the microbial community structure.
So, are we what we eat? Sorta. This was an extremely simplified system; thankfully our diets are more than corn oil, corn starch, casein, sucrose, and mashed peas, and we have thousands of different microbes in our guts. However, despite its simplicity, this study suggests that we can alter our gut microbiota at will. This can be medically important. For instance, the amino acid fermenting bacteria associated with the high protein diet are also associated with a variety of inflammatory syndromes; it would be nice to reduce their relative numbers.
However, another study reminds us that our family background is also a significant factor in determining our personal microbial community structure, and our health. Using mice, a group of researchers from Nebraska found a correlation between certain regions of DNA and certain types of bacteria in the gut.
Genetecists use the term “Quantitative Trait” to discuss something that can be measured (quantified) and inherited (trait). So, height is a quantitative trait. Once you have a quantitative trait, you can look for genes that influence it. In the case of height, there are dozens of such genes, each of which makes a small contribution to overall height. Each gene occurs in a specific region, or locus, in the DNA, so a geneticist could refer to a "Quantitative Trait Locus" (QTL) associated with height. It's useful to note that a QTL is not a gene; it's a region of DNA, potentially carrying many genes, associated with a particular trait.
The researchers decided to treat the composition of the gut microbiota as a quantitative trait. They did this by making a mathematical model that combined the relative abundances of 64 different microbes into one measurable variable; you could measure the relative numbers of these 64 microbes in the poop of any mouse, and compare it with any other mouse. Given the existence of so many different laboratory strains of mice, this made for a lot of data to analyze.
The big question, then, was whether there were any genetic loci that corresponded with variation in this quantitative trait. There were. At least 13 different places in the mouse genome corresponded with definite variations in their gut microbiota. If two mice had different versions of the same gene in one of these places, then they would likely have different ratios of different microbes in their guts.
So what genes determine what microbes we have? The answers are still a bit vague; we know that certain regions of the DNA are associated with these traits, but each region has several genes. Nonetheless, there are some cases where there are candidate genes that really stand out. For instance, there’s one QTL associated with increased numbers of two specific types of bacteria; this QTL contains genes specifically involved in controlling immune responses in the gut.
This study is highly suggestive, but not conclusive. It establishes a correlation, but the results were all retrospective; no predictions were tested. For example, it would be great to see a cross of two different strains of mice predicted to favor certain different bacteria. Would it be possible to accurately predict what bacteria the offspring would favor? Even without this bit of proof, this study represents a buttload of work, and a very promising entrée into a challenging question. Like the study with gnotobiotic mice, this study also has relevance to health and medicine, as it may explain the observed linkage between certain QTLs and diseases such as Crohn’s disease.
This research also raises a big question in the symbiosis between mammals and our gut microbiota. The authors of this study use the link between certain immune system genes and certain microbes to support the argument that the immune system evolved for the purpose to maintaining favorable microbes in the gut. However, I’m a microbiologist, and I look through the other end of the telescope. To me, this is further evidence that certain microbes have been exerting selection pressure upon us, in order to make a better environment for themselves.
Either way, your doctor’s advice for good health and longevity remains much the same: eat right, and choose good parents.
Andrew K. Benson, Scott A. Kelly, Ryan Legge, Fangrui Ma, Soo Jen Low, Jaehyoung Kim, Min Zhang, Phaik Lyn Oh, Derrick Nehrenberg, Kunjie Hua, Stephen D. Kachman, Etsuko N. Moriyama, Jens Walter, Daniel A. Peterson, and Daniel Pomp (2010). Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proceedings Natl. Acad. Sci. USA 107: 18933-18938.
Jeremiah Faith, Nathan P. Mc Nulty, Federico E. Rey, Jeffrey I. Gordon (2011). Predicting a Human Gut Microbiota’s Response to Diet in Gnotobiotic Mice. Science 333: 101-104.
Thursday, December 16, 2010
Buddy Breathing
We all need to breathe. This is true for us humans, and it’s true for most microbes. However, many microbes have different approaches to breathing. A new discovery from Derek Lovley at University of Massachusetts reveals surprising flexibility in microbial respiration—and how a couple of bacteria can cooperate and evolve to meet a challenge to their survival.
When we breathe, we’re consuming oxygen; we need this oxygen to oxidize our food, which is mostly carbon and hydrogen (think “carbohydrates”). We often think of oxidation as simply “reaction with oxygen”. After all, we are taking the carbon and hydrogen we eat and making it into carbon dioxide and di-hydrogen oxide (or water, for the less nerdy). However, there is a more precise definition of oxidation that appeals to chemists and microbiologists. Oxidation is the loss of electrons by an atom; since the electrons have to go somewhere, they are accepted by another atom, which we say is getting reduced.
When we respire (which is why we breathe), we are taking electrons away from the carbon and hydrogen in our food, and giving those electrons to oxygen. We get energy from this process, which we use to make the ATP that powers the cell. But Bacteria and Archaea have figured out how to take electrons from carbon and hydrogen and give them to atoms other than oxygen: sulfur, iron, arsenic, and all manner of other atoms. They don’t necessarily get as much energy out of these processes, but it’s better than dying if no oxygen is available.
One example of this type of anaerobic (no-oxygen) respiration is seen in the Bacterium Geobacter metalloreducens, which, as its name suggests, reduces metals. When it respires, it “eats” ethanol, taking away its electrons, and “breathes” metals such as iron, giving them electrons and producing rust. Another example of this type of respiration is found in the related bacterium Geobacter sulfurreducens. It can’t eat ethanol, but it can eat hydrogen. It can’t “breathe” oxygen or iron, but it can give electrons to sulfur (as the name suggests) or the compound fumarate.
Whether or not oxygen is used for respiration, the basic outline of the process is the same: electrons are plucked off of the food, and go through a series of intermediate carrier molecules before they end up at the terminal electron acceptor. The whole process takes place in the cell membrane: the electrons on their carriers never leave the cell until they are handed to the terminal electron acceptor. As the electron is passed from carrier to carrier, it releases energy, which is used to make ATP.
The outline of this process is the same for us, and for Geobacter metalloreducens and for its cousin sulfurreducens. However, metalloreducens and sulfurreducens are specialized for particular combinations of food and electron acceptor: metalloreducens likes ethanol and iron, while sulfurreducens likes hydrogen and fumarate.
But what happens if you put these two organisms in an environment with food for only one, and an electron acceptor for only the other—ethanol and fumarate? It would seem that metalloreducens could take electrons from ethanol, but not get rid of them, while sulfurreducens could give electrons to fumarate—if only it could obtain some electrons from somewhere!
Fortunately, these two different types of cells can cooperate. Geobacter metalloreducens can get rid of electrons from ethanol—it just won’t get much energy out of the deal. Water, it turns out, is mainly H2O, but always has some OH- and some H+; metalloreducens can give those electrons from oxidizing ethanol to H+, making hydrogen gas. This works out nicely, since sulfurreducens can “eat” the hydrogen, and give the electrons to fumarate. So, even though each member of the partnership only gets half as much energy, it’s better than getting no energy at all.
This type of collaboration is called “syntrophy,” and it’s actually fairly common in the microbial world. One organism respires, producing hydrogen; the next organism respires, “eating” that hydrogen. Each organism has a complete respiratory system, but the two respiratory systems are dependent upon each other. Syntrophy occurs in your own personal digestive system, in which one organism makes hydrogen, giving electrons to another organism, which uses them to make the methane that makes our farts flammable. Up till now, when syntrophy has been studied, it has always involved hydrogen as the electron carrier. However, Lovley’s group introduced an interesting complication to this process.
Lovley’s group mutated sulfurreducens so that it absolutely can’t eat hydrogen. They then started the experiment again, giving a mixture of metalloreducens and sulfurreducens nothing but ethanol to eat and nothing but fumarate to breathe. Given that the options for these populations of cells were (a) evolve or (b) die, it’s unsurprising that option (a) was chosen.
What was surprising was the way that evolution worked. Rather than jury-rigging a way for sulfurreducens to eat hydrogen, evolution chose a method that involved the direct transfer of electrons from metalloreducens to sulfurreducens. I mentioned that when a cell respires, the electron gets shuttled from one carrier molecule to another as it moves from the “food” to the electron acceptor. These carrier molecules stay in the cell membrane, and respiration happens strictly within the confines of the cell.
What Lovley found was a mutant form of sulfurreducens that produced electron carriers that could be exported to cells of metalloreducens, pick up electrons, and then shuttle back to sulfurreducens. The sulfurreducens cell had to make filaments for these carriers to travel along, and had to grow next to metalloreducens—but one part of respiration was happening in one cell, and the other part of respiration was happening in another cell. This is syntrophy, but without using hydrogen to transfer the electrons. Electrons were flowing directly from one cell to the other.
Being thorough, Lovley’s group made sure that they were seeing electrons moving directly between the two cells. They checked to see if hydrogen was being exchanged—it wasn’t. They found the genes in sulfurreducens that had mutated to allow this to happen, and deleted them—and growth stopped. They looked for electrical current between cells—and found up to four microamperes of electron flow. They looked at the cells using various forms of microscopy, and found that they grow in close contact…
(this is a clump of cells, with metalloreducens stained green and sulfurreducens stained red. They can't grow separately, like the cells in a normal syntrophic relationship.)
…and they used the much higher magnification of electron microscopy to show that sulfurreducens made a web of filaments that connected it to metalloreducens cells, and that electron carriers (visible as dark spots) could move along those filaments:
This has never been seen before—respiration has always been contained within one cell. But now, we know that the process of respiration can be completely shared between two cells, a sort of bacterial “buddy breathing.” One cell oxidizes food (metalloreducens taking electrons from ethanol) and the other reduces an electron acceptor (sulfurreducens giving electrons to fumarate). This kind of metabolic sharing allows both cells to survive in an environment that could not sustain each individual, and so gives life the ability to exploit ever more of the earth’s environment. It’s also a nice example of life’s seemingly endless creativity—what Daniel Koshland calls “improvisation”—using mutation and selection to try out novel solutions to novel problems.
Zarath M. Summers, Heather E. Fogarty, Ching Leang, Ashley E. Franks, Nikhil S. Malvankar, and Derek R. Lovley (2010). Direct Exchange of Electrons Within Aggregates of an Evolved Syntrophic Coculture of Anaerobic Bacteria. Science 330: 1413-1415.
Koshland, Daniel (2002). The Seven Pillars of Life. Science 295:2215-6.
Tuesday, July 13, 2010
Our parasites, our selves
In addition to the trillions of bacteria in our gut, there are other residents of our intestines that we have evolved with—the myriad types of worms that parasitize us. Given the deservedly bad reputation of whipworm, ringworm, tapeworm, schistosomes, and their helminthic ilk, it comes as a surprise that the last ten years of research indicate that these worms can actually do us some good.
Our immune systems evolved in a world of worms; the worms evolved in the world of our immune system. For a worm to survive in our gut it has to somehow survive the assault of our immune system, and so evolution has favored those worms that have figured out how to tame an immune response. For us to have survived with such parasites, our immune system evolved with the assumption that it’s being restrained. If, with modern hygiene, the reins are off the immune system, it can run wild. So, we in the modern western world are far more likely to have allergic reactions and autoimmune disorders. If you can stomach it, you can now effectively treat your allergies not with antihistamines, but with intestinal worms purified from other peoples’ poop.
So far, most of the research on the interaction between human and worm has focused on the human half of the equation—exactly what immune cells are being stimulated and suppressed by the worms. People are now trying to understand the worms’ point of view.
Worms infect our guts, and use the nutrients that they steal from us to produce millions of eggs, which we spread by defecating. These eggs can infect another human when the eggs are eaten; it's polite to say that it’s oral-fecal contamination, but really it’s just getting stuff in your mouth that has some s#!t on it. The eggs don’t want to hatch until they are in the human gut, but what I find surprising is that the eggs do not recognize the human gut. The way they know that they’re in a human is by recognizing the bacteria that live in our gut. It's only when they see the right bacteria that they hatch (here's the movie):
A group of researchers at the University of Manchester studies whipworm, and tried hatching whipworm eggs in vitro. Initially, they found that the eggs hatched only if there was some material from the host animal’s gut present. Using a microscope, they found that the ends of the eggs that were ready to hatch were covered in bacteria (here treated so that they glow green).
Some further experimentation showed that most of the inhabitants of the animal gut worked to trigger egg hatching, and that direct contact between the egg and the bacteria was absolutely necessary for hatching. But what is the nature of the interaction between bacteria and egg? Intestinal Bacteria produce a variety of protein structures that allow them to stick to their hosts’ cells. The “fimbriae,” or fringes, are little filaments of protein with extra-sticky proteins at their ends:
Bacterial cells that couldn’t make fimbriae, or those that had their fimbriae covered up, were both incapable of triggering egg hatching. So, that’s the bacterial side of the interaction; the worm’s egg side remains a little hazy, but the researchers guess that there is a receptor on the egg that is triggered by the fimbrial protein.
One cool aspect of this study is that they were able to show that this relationship has real-world significance. The researchers used mice, and treated them with strong antibiotics to remove most of the bacteria from their guts. After two days of this treatment, the mice were infected with whipworm eggs. After 18 days, they had about a third as many intestinal parasites as their siblings who had not been given antibiotics. That’s the good news; the bad news is that they have a rather extreme immune response to the worms, probably on account of not having enough bacteria in their guts. And of course, if worms do become established, they further regulate the immune system.
Hygiene is good—nobody argues for a return to the bad old days, in which pretty much everybody had multiple infections and suffered the harm they caused. But allergies and autoimmune diseases are bad. Hopefully, we will eventually understand the complicated evolutionary ménage a trois of ourselves, our bacteria, and our worms, and we can reach an equilibrium without worms or dust allergies.
K. S. Hayes, A. J. Bancroft, M. Goldrick, C. Portsmouth, I. S. Roberts, and R. K. Grencis (2010). Exploitation of the Intestinal Microflora by the Parasitic Nematode Trichuris muris. Science 328:1391-1394.
Picture of Fimbriae from Brock Biology of Microorganisms