Sunday, June 24, 2018
What’s my pre-Cambrian rabbit? What’s YOUR pre-Cambrian rabbit?
Tuesday, April 17, 2012
My attempt to teach the controversy: adaptationist vs stochasticist
This month, Tennessee enacted a law that would require education officials at the state and local level to
assist teachers to find effective ways to present the science curriculum as it addresses scientific controversies…[and] help students understand, analyze, critique, and review in an objective manner the scientific strengths and scientific weaknesses of existing scientific theories [such as] biological evolution, the chemical origins of life, global warming, and human cloning.
These “teach the controversy” laws are a nod and a wink to creationists and global warming deniers, denizens of that parallel world I visited earlier. Meanwhile, in the reality-based community, we have some really fun studies that engage real questions about evolution.
One of the long-standing tussles in evolutionary theory is about what pushes evolutionary change. In one corner we have the adaptationists: folks such as Richard Dawkins (and many microbiologists) who see good old-fashioned Darwinian natural selection as being the main force behind change. In the other corner we have the stochasticists: folks who argue that chance fluctuations and genetic drift in small populations are the main way that changes get established within a population (the blog Sandwalk is an example; to some degree, Stephen Jay Gould was of this school).
Evolutionary biologists of both stripes have long known that islands are prolific incubators of unique species. The Galapagos Islands are the classic example, but pretty much any island chain will have its oddities. My favorites are California’s Channel Islands, with the world’s second cutest fox species and the oxymoronic pygmy mammoth. Adaptationists will say that the unique, isolated environment of any island will drive evolution of founder species in idiosyncratic directions, producing species perfectly and uniquely adapted to every specific island. Stochasticists will say that island species are typically “founded” by a very small number of individuals, and since no individual perfectly represents a species, the differences between island species are due to these “founder effects.”
If you were a god, the question of who is correct here would be easy to solve—simply find some islands with distinctive (but related) species, and reverse time’s arrow until you arrived at the common ancestor of all the different types. Being merely human, though, you could do what a group of researchers from Harvard, Duke, and UC Davis did, and act like a god to a bunch of lizards.
When I was a kid, I was a god to a lizard; it was a Carolina Anole that we bought at the Los Angeles County Fair, where it was sold as a “chameleon” because of the species’ ability to change color from muddy brown to green. Anoles are native to the islands of the Caribbean, where, over millions of years and hundreds of islands, they have diverged into many different species. Why so many different species? Are the individual islands all so different that they select for unique adaptations, or are we seeing the effects of chance events in small populations?
To play god for these lizards, the researchers took advantage of an "act of god". A hurricane had eliminated the lizard population of seven tiny islands in the Bahamas. The researchers repopulated each of these lizard-free islands with an anole Adam and Eve, chosen at random from a large and well-established population on a nearby island.
For the stochasticists, this is a promising start. Given that each island’s Adam and Eve were randomly chosen, some of the islands would start with larger-than-normal founders, and others would start with smaller-than-normal founders. Stochasticists would believe that subsequent generations would bear the signatures of their founders, and that you could tell many generations later whether an island’s population had been founded by a relatively large or small Adam and Eve.
What about the adaptationists? The godlike researchers had something to satisfy them as well. It’s pretty well established that small shrubs—like those found on the new islands—provide a selective advantage to anoles with shorter limbs. The Adams and Eves chosen to populate these islands came from a population that had evolved with big trees, and so tended to have long limbs. Thus, all the new populations were subject to selection pressure to make their limbs shorter with every generation. Adaptationists would believe that, after a few generations, all the anoles on all the islands would have the same short limbs, regardless of which Adam and Eve founded their island’s population.
Part of being godlike is being patient, so the researchers gathered data on these lizards for four years; the populations of the islands grew from their founding couples to thirty or forty lizards, all of whom were subject to annual measurements of limb length and other characteristics. All the lizards also gave DNA samples to be sequenced, giving the researchers a certain godlike omniscience about these beasties.
So, after four years, what did the researchers find? Were the stochasticists right, and the lizards descended from larger Adams and Eves remain larger? Or, were the adaptationists right, and all the lizards got smaller and smaller limbs?
Of course, as one of my teachers would say, absolutists are always wrong! After four years, all the lizard populations had clearly evolved by selection. On every island, the population’s limbs were all shorter than the limbs of that island’s Adam and Eve. However, after four years, the imprint of the founders was still visible—the larger the founding couple’s limbs, the larger their descendents’ limbs. In one potent, information-packed graph, we have this result about the last years of the study:
(The islands have poetic names like "N1" and "N15"; all populations evolved towards shorter limbs, and generally speaking, larger-limbed founders lead to larger-limbed descendants. Just to be sure, the researchers followed the large population that provided each island’s Adam and Eve, and it barely changed over this time.)
There’s two other things that the lizard gods noticed in their study. One thing fit in very nicely with the stochasticists’ world-view. The researchers measured the amount of variation in some of the lizards’ DNA sequences (they looked specifically at non-coding DNA, a sort of filler that is not essential or even useful to the lizard; all animals have lots of this, and by definition it is not affected by selection, only by stochastic events). In the generations of Adam and Eve and their first offspring, the lizards on each island had a lot of variation in their DNA sequences. However, in succeeding generations, this sequence variation collapsed. The populations experienced a genetic bottleneck, and all the subsequent generations showed the effect—essentially, they became highly inbred. This fits with the stochasticist view of how island populations evolve and become different from one another, as each population’s genetic variation collapses around a different focus.
Another observation bolsters the adaptationist viewpoint. Most of the lizard populations peaked after three or four generations, and were actually in a significant decline as the study ended. This is a phenomenon that would be unsurprising to the Rev. Thomas Malthus: a small population in a rich environment expands, and eventually exceeds carrying capacity, leading to a crash. Under such circumstances, the selection pressure gets turned up to eleven.
The authors of this paper are not godlike enough to see into the future. Really, they studied just the very beginning of the island evolution process, and nothing like speciation has happened yet. The authors suggest that the adaptationist trend will dominate in the short term (especially given the pressure of overcrowding), driving all the populations to a similar conformation. However, beyond that they are agnostic: there aren’t any significant environmental differences between the islands (at least, any that they can perceive), so selection will no longer work as a force to make the island’s populations divergent. Also, the islands’ populations are really small, so it’s relatively easy for a chance event to have a profound effect. Retreating from the role of omnipotent and omniscient gods, they assume the mantle of Solomon, and judge the case of stochasticist v adaptationist by cutting the baby in half.
Kolbe, Jason J., Manuel Leal, Thomas W. Schoener, David A. Spiller, and Jonathan B. Losos (2012). Founder Effects Persist Despite Adaptive Differentiation: A Field Experiment with Lizards. Science 355: 1086-1089.
Sunday, April 1, 2012
Apparently, Bacteria have ears...
Bacteria have an endless ability to amaze. Even plain old Escherichia coli, the workhorse organism of thousands of labs—an organism that, in my youth, I thought too boring and well-known to be worth focusing a career on—still generates research that leaves us dumbstruck and groping for explanations. Nowadays, as often as not, these surprises turn up on the fringes of science and don’t really affect our understanding of how the world works. However, they do make us pause and scratch our heads—sometimes at the result itself, other times at why the initial experiments were done. So, we have findings from Czech researchers about how E. coli in “heavy” water (enriched for the deuterium isotope of hydrogen, 2H2O) grow faster than they do in normal water. We have the finding from an astrobiology group that growth in zero gravity causes changes in the expression of almost half of E. coli’s genes. And now, a new report suggests that E. coli can actually sense and respond to sound—in a word, bacteria can hear.
The finding, like many in the history of science, is an example of Pasteur’s dictum about fortune favoring the prepared mind. In this case, the mind belongs to Felix Balatro of Miskatonic University. He was actually testing a pet hypothesis about whether Bacteria could learn.
Of course, one would not expect terribly deep thinking from a bacterium. Nor would you expect deep thinking from the flatworm Planaria—however, this has been demonstrated using a T-maze. A T-maze is the simplest possible maze: the worm can go forward from the bottom of the T to the top, then it can turn left or right. If the reward is consistently on the right, then the little guys start turning right more than half of the time. Balatro had the rather crazy idea that bacteria could do the same trick. One problem he had to solve was a matter of scale: a planarial T-maze is a few centimeters long, a distance that would take a couple of generations for E. coli to swim. Fortunately, advances in microfluidics and techniques borrowed from semiconductor production enabled him to produce a T-maze of millimeter scale, with exactly equal concentrations of an attractant (aspartate) at each branch of the maze.
Now, the first piece of luck for Balatro was that he didn’t really think this through. Planaria, being flatworms, have a clearly defined left and right. They crawl, instead of swimming. So, the right side of the T-maze corresponds only to the right side of the flatworm’s body. Not so with E. coli. They swim, and spiral and loop and frequently tumble as they swim, so they “know not their right hand from their left.” Anyone knowing this basic fact of E. coli’s biology knows that this experiment would fail—but Balatro, either through ignorance or (more likely) stubbornness, pushed ahead.
Amazingly, E. coli seemed to learn to turn right. Balatro ran his cells through the T-maze, and collected the cells that made it to the right side of the maze. Using some nifty microfluidic devices, he let these cells reproduce for one generation, and ran them through the maze again. Again he selected the cells that turned right, and let them grow again…and again, and again, for approximately a thousand generations. After this time, when he ran a thousand cells through the maze, he found that between 60 and 70% of the cells would turn right.
This result is completely, totally, 100% unbelievable. Being a good scientist, Balatro didn’t believe it. Suspecting some barely perceptible turbulence in his test chamber, he turned the microfluidic slide upside down in the microscope—and obtained the same result. He wondered if the bacteria were somehow responding to light, so he blacked out his lab and ran the cells through the maze with the microscope’s lights out—and again found that nearly three quarters of the cells turned right.
Magnetotaxis—the ability to sense and respond to the Earth’s magnetic field—is not unknown among the bacteria, so Balatro tried to test this. “Right” in his original maze corresponded to south-southeast. He spent a day rearranging the tangle of pumps and controllers surrounding the apparatus so that “right” now corresponded to north-northwest, and he must have experienced a mixture of surprise and elation when he found that now, 75% of his cells got to the T and turned left!
Balatro’s celebratory mood was presumably deflated when he shared his results with his colleague, D. Avril Poisson. She looked through the microscope at the maze, coolly removed a magnet from a nearby refrigerator door, and set it upon the stage. The resulting magnetic field was many times stronger than the Earth’s, and the bacteria did not change their behavior at all. In fact, when Balatro ran the experiment in her presence, the bacteria seemed to forget everything—they got to the T, and half turned left, half turned right.
Here’ was Balatro’s second piece of luck. It had to do with a combination of his intellect and his personal eccentricities. I got to know one of Balatro’s PhD committee members when I was in graduate school, and she described him as “one of the most brilliant and…um…uh…ehhh…I guess you could say lateral thinkers I’ve met.” He apparently lived in the lab, only going home to shower; he spent a fair amount of his time in lab stoned, and he spent absolutely all of his time in lab, awake or asleep, with the stereo blasting the Grateful Dead at deafening volume. He even ditched one thesis advisor (and, thus one field of study and one future career) for another solely because his newly chosen advisor was deaf and didn’t mind the noise—and so oncology’s loss was microbiology’s gain.
Rather than mope about a failed experiment, Balatro realized that only one thing had changed when Poisson had entered the room. Out of hard-learned politeness, he had muted Jerry Garcia’s solo from “Sugaree” at the Rockpalast, 1977, blasting from the speakers in the south end of his lab. Nothing else had changed—nothing about light, the magnetic field, temperature, pH, nutrient or redox gradients, or any of the myriad other things bacteria are known to sense. Just pure, Gratefully Dead sound. Had he discovered phonotaxis?
Of course, Balatro confirmed this result, and spent a lot of time designing experiments that eliminated other possibilities and left the conclusion that his bacteria could hear and turn towards sound. And so we have the paper, given the innocuous title “Novel functions for mechanosensitive channel proteins in an evolved Escherichia coli system,” and slipped into print in the obscure journal Acta Pathologica, Microbiologica et Immunologica Scandinavica. It’s as if Balatro doesn’t want us to notice that he has discovered something incredible.
However, this is a powerful paper; Balatro, working with Poisson, even proposes a plausible mechanism. Sound, at the cellular level, is a mechanical phenomenon—an alternation of high and low pressure. Bacterial cells have mechanosensory proteins in their membranes, and these have been intensively studied for their role in helping cells survive osmotic stresses. These proteins, as they sit in the cell’s membrane, are deformed when the cell membrane is deformed; the change in the protein’s shape causes the protein to send a signal into the cell, resulting in a change in the expression of several genes.
Balatro and Poisson examined the genes for these mechanosensory proteins, mscL and mscS, in the cells that consistently turn towards high-intensity sound, and found that these genes had evolved over the course of generations of selection. The genes had, in fact, recombined with genes for chemotaxis—that is, genes that encoded proteins that sensed food, and directed cells to swim towards higher concentrations of food. The evolved gene encoded a hybrid protein that still sensed mechanical pressure (though this too had changed slightly), but now controlled the cell’s swimming behavior.
This result is curious, and leaves open lots of questions. We still don’t quite understand how sound waves, which should be much larger than a bacterial cell, can have a perceived direction to E. coli—it would be as if we could tell, just using our own senses, what direction a change in barometric pressure came from. There is also the puzzle of how the changes in the pressure-sensing component of the MscL and MscS proteins in these “hearing” bacteria relate to their ability to hear. Information about the structure of these mutant proteins should provide some answers, and Balatro is quite able to get that data rapidly.
Ultimately, like cells growing in Deuterium or microgravity, this is a completely artificial situation, and thus not likely to be of especial biological significance. Still, it’s pretty neat, and a good reminder of both how science progresses and how amazingly extraordinary even “ordinary” bacteria can be.
Mann, L.R., and Moses, V. (1971). Properties of Escherichia coli grown in deuterated media. Folia Microbiol Praha 16 (4): 267-84.
Rosenzweig, J.A., Abogunde, O., Thomas, K., Lawal A., Nguyen, Y-U., Sodipe, A., and Jejelowo, O (2010). Spaceflight and modeled microgravity effects on microbial growth and virulence. Applied Microbiology and Biotechnology 85 (4): 885-891.
Jacobson, A.L, Fried, C., and Horowitz, S.D. (1966) Planarians and memory. Nature 209: 599-601.
Matsunaga, T., and Okamura, Y (2003). Genes and Proteins involved in Bacterial Magnetic Particle Formation. Trends in Microbiology 11(11): 536-41.
Balatro, Felix, and Poisson, D. Avril (2012). Novel functions for mechanosensitive channel proteins in an evolved Escherichia coli system. Acta Pathologica, Microbiologica et Immunologica Scandinavica 120 (4): 4652-4661.
Haswell, Elizabeth, Phillips, R., and Rees, D.C. (2011) Mechanosensitive Channels: What Can They Do and How Do They Do It? Structure 19: 1356-1369.
Tuesday, March 20, 2012
A lesson from Botox
There's not much in common between what the Real Doctor studies--ophthalmology--and my chosen field of microbial genetics and physiology. One of these rare commonalities is an interest in Botox. For the Real Doctor, it's a handy tool to paralyze an ocular muscle and cure a case of walleye. For me, "Botox" is botulinum toxin, a poison secreted by Clostridium botulinum, and a nice example of how bacterial toxins work.
Right off the bat, I gotta say that bacterial toxins are amazing. It always gives me pause when I see examples of evolution reaching across domains of life. I mean, it is not too surprising when a bacterium evolves a chemical signal to communicate with another bacterium--after all, they share the same biochemistry. But with toxins, bacteria have evolved a very complicated set of molecules that target very specific proteins on the surface of very specific nerve cells in only a few types of organisms; the bacterium is reaching across domains to communicate biochemically with an alien biochemistry.
That said, there are other reasons to be interested in bacterial toxins. They’re amazingly potent—30 nanograms of botox, or a volume of about one billionth of a sugar cube, is enough to kill a human. Their power and specificity makes them valuable tools for understanding the biochemical processes of our own cells, and some (such as botox) have medical uses. Bacteria, in their diversity, have evolved a huge number of bacterial toxins specific to different tissues in different hosts, but most of these toxins are variations on a couple of themes.
One major class of bacterial toxins can be thought of like nuclear missiles; they require two sophisticated components to do their deadly job. By itself, an A-bomb is not so useful a weapon--unless you deliver it to its target, you will only damage yourself. By itself, a guided missile won't do too much damage to its target--it's merely a bus to deliver the payload. But put together the A-bomb and the bus, and you have yourself a tremendously destructive weapon. The bacterial "A-B" toxins are the same way. The "A" component of these toxins is like the A-bomb--a tremendously destructive protein molecule, but it needs to be delivered to its target. The "B" component is like the missile (or bus), a protein that is by itself harmless, but protects the "A" component and delivers it to its target.
Botulinum toxin is an example of an A-B toxin, with a wicked but delicate A component and a very interesting B component. The bacteria release this toxin in our guts, an extremely acidic environment, and it must travel into our blood and then find a specific molecule on a specific type of nerve cell to do its dirty work. Some recent work by a group at the Sanford-Burnham Medical Research Institute in La Jolla, California, has given us a peek into how the B component delivers the destructive A component to its target, and also--like any good study--raised some more interesting questions.
The researchers focused on the first stage of this deadly missile's trajectory--the trip from the acidic digestive system into the more neutral bloodstream, a hazardous journey that almost no proteins can survive. As long as people have known that botulinum toxin is made of protein, it has been a mystery how it avoided destruction. So, what about B allowed this? By making various modified versions of the B component, they found that only one of the four parts of this carrier was necessary for protectin the A component from acid. Then, since the function of any protein is intimately tied to its structure, they tried to find out the structure of this minimal B and how it fit together with the A. They found a couple of interesting surprises.
First, the B protein is sensitive to acid, just as susceptible to acid as the delicate A component of the toxin. Both B and A, individually, are attacked by acid at a few specific sensitive places, and break into a few distinctive pieces. However, if you put them together in an acidic environment, they laugh it off. The reason is in how these proteins fit together: all the bits that are sensitive to acid are covered up by the way the proteins nestle up against each other. They snuggle against each other to protect each other from harm, a romantic image if we weren’t dealing with deadly poisons.
A second interesting feature came to light when the researchers looked at exactly how these proteins fit together. The researchers were able to recognize the specific parts each protein that touched each other. These parts were notable because of how they responded to acidity: in the harsh of environment of the stomach, these parts of the proteins remained neutral, and helped to hold the A and B complex together. However, when the protein complex was moved to a neutral environment, these parts of the protein turned acidic--enough that they would cut the bonds between the A and the B proteins. The connections between the A and B proteins acted like the explosive bolts that hold together the stages of a missile, holding them together through the boost phase but dramatically separating the two when the booster is done. Indeed, the researchers conclude that this is how the first part of botulinum toxin's journey goes: the minimal part of the B component protects the A component in the stomach and carries it into the bloodstream. Once the two are in the bloodstream, the neutral environment causes B to cut its links to A, and the A component can go on its merry way.
This is all pretty cool. It solves a mystery about how botulinum toxin works, and actually suggest as way that biotechnologists could design protein-based drugs that could be taken orally. If a protein drug could be designed to fit with a carrier similar to botulinum toxin B component, then it could survive the trip through the stomach and be absorbed into the bloodstream, where it could do its job.
However, for my money, the most surprising (and instructive) thing revealed by the structure of the minimal botulinum toxin is that the "A" and "B" components have almost identical structures. Proteins are linear strings of hundreds of amino acids, and these strings clump and fold together in unique and characteristic ways. The structures are often quite complex, with enough helixes and turns and twists that a single protein's structure would resemble a plate of spaghetti, the the entire platefull were one loooong noodle. It is this unique molecular shape that gives each different type of protein the ability to do its specific job--say, disable a nerve cell for the A component of botulinum toxin, or protect another protein from acid for the B component. It is surprising that two proteins that you might expect to have structures as different as an A-bomb and a booster rocket to look almost the same.
Check out this picture: the A component is an orange stringy ribbon, and the B component is a green stringy ribbon. The two structures are superimposed upon each other, and you can see that they are almost identical. (This is just the first third of each of the proteins, but the remaining 2/3 are available, in stereo, at http://www.sciencemag.org/content/335/6071/977/suppl/DC1)

One of the great big hairy problems of modern biology is how proteins--linear molecules--fold up into unique three-dimensional shapes, and botulinum toxin gives us another bracing surprise here. I teach my intro bio students that the "primary structure," or linear arrangement of amino acids in the protein, determines the three-dimensional structure of the protein. A change in the amino acid sequence should result in a change in the three-dimensional structure. I'd expect the botulinum toxin A and B components to have very similar amino acid sequences, given their structural similarity. However, their amino acid sequences are only 20% identical. That's crazy.
The authors, being most interested in biochemistry, don't pursue a Big Question raised by this--how does this evolve? The genes for botulinum toxin A and B components are probably also about 20% identical (mea culpa--I don't have my computer with me, so I have a hard time looking this up. It is left as an exercise for the interested reader). It's possible that two entirely different genes evolved to have such similar structures, but I don't think it's at all likely. More likely, it is a case of an ancestral gene being duplicated, and then each of the two copies undergoing evolutionary change (this hypothesis is weakly supported by the observation that the A and B genes tend to be clustered). These changes altered the sequences of the proteins, while preserving their three-dimensional structures. This would be like sitting down with a sonnet and a thesaurus, and changing 8 of every 10 words. If you were careful, you could preserve the structure, meter, and rhyme scheme of the sonnet, while completely changing the meaning of the poem—and a textual analysis would show no relationship between the source and the end product.
If I could tell these researchers what to do, I would have them gather more structure and sequence information from a variety of related toxin proteins. It would be fun to make a family tree, and see just how much structure could be conserved as amino acid sequence changes.
Shenyan Gu, Sophie Rumpel, Jie Zhou, Jasmin Strotmeier, Hans Bigalke, Kay Perry, Charles B. Shoemaker, Andreas Rummel, Rongsheng Jin (2012). Botulinum Neurotoxin Is Shielded by NTNHA in an Interlocked Complex. Science 335: 977-981.
Peck, MW (2009). Biology and genomic analysis of Clostridium botulinum. Advances in Microbial Physiology 55: 183-265.
Tuesday, August 23, 2011
When 3.4 billion years old you reach, look as good you will not
I finally got a chance to read the latest microbial news that is creating a buzz, the discovery of some tantalizing maybe-fossils of cells over 3.35 billion years old. (News reports here and here; a couple of blogs’ opinions here and here.) I’m not a geologist, so I can’t adequately judge everything about the paper, but as a microbiologist I can offer my two cents.
I’ll confess that I rolled my eyes a bit when I first heard the news, for several reasons. First, I’m not a fan of science by press release. Fortunately this is actually in the legit literature*. Second, the topic of ancient microfossils is one that leads to a lot of squinting at grainy pictures and seeing what one wishes to see. Third (and partly because of the very subjective nature of interpretation) this has been a field of heated, occasionally quite personal debate, and I am conflict-averse. Finally, I’m not sure it matters.
Here’s the problem. It would be nice to find a beautifully preserved, clearly cellular structure over 3 billion years old with unambiguous evidence of biological functions. It would also be nice if a unicorn came by and pooped golden bricks for me, and about as likely. First, it’s really hard to find nice sedimentary, fossil-bearing rocks that are that old—plate tectonics being what it is, any really old rocks are likely to have been recycled, squeezed and cooked so much that fossils wouldn’t exist. Second, any life present over 3 billion years ago would have been unicellular and very small. It’s hard to find small things. Finally, it’s hard to find something that old that is unambiguously biological. Non-biological processes can generate mineral patterns that look cell-ish, and even mimic some of the chemical processes of life. Remember the martian meteorite that was supposed to have evidence of life on Mars?
So, here’s this report from Martin Brasier and his associates at the University of Western Australia. It’s actually pretty good.
To solve the first problem, they found one of the few places on earth with really old, relatively un-metamorphosed rock—the boonies of Western Australia. These are sandstones, sedimentary rock that was laid down on top of some volcanic rock that’s about 3.5 billion years old, then buried by more volcanic rock 3.35 billion years ago. That this rock is sandwiched between these layers is nice—the volcanic stuff is relatively easy to date, and it helps to protect it from modern organisms (there are some bacteria that live in surprisingly deep hot basalts; this geological setting minimizes the chances of contamination by these organisms, but does not eliminate it).
To solve the problem of finding fossils, they looked in likely places—places where carbonaceous traces were present. Here’s some pictures of what they found:
These are, of course, not convincing on their own to the layman or the specialist. Which brings up the third problem, and the meat of the report. As the authors say with modest understatement, “determining the biogenicity of putative [3 billion year old] microfossils is notoriously difficult.”
The authors provide some pretty good structural arguments. The candidate microfossils are cell-shaped, cell-sized (unlike the supposed martian fossils), and (like modern cells, but unlike some geological artifacts) quite uniform in size. They are located in the sediments like normal cells would be in such an environment. Some pretty amazing work with spectrometry shows that the things that look like cell walls are actually where most of the carbon is found. So, the geology is good and the taphonomy (I love that word—it means the conditions of fossil formation) is pretty good.
Some more convincing evidence comes from the chemistry of the supposed fossils. The researchers determined not just that carbon and nitrogen and sulfur were present, but which isotopes of carbon and sulfur were present. (Isotopes are versions of atoms with different numbers of neutrons—the number of protons and electrons remains the same, but an atom may have more or fewer neutrons. So, most carbon has 6 neutrons per atom, but a very small amount of carbon has 7 neutrons per atom.)
Isotope fractionation is one of the more reliable fingerprints of life. When living things such as plants and microbes take carbon dioxide from the atmosphere and make it into part of a living thing, they preferentially take the certain isotopes of carbon. To “fix” carbon dioxide, a biological enzyme first has to catch a molecule of the gas. The enzyme is not all that great at this, so it tends to catch relatively more of the heavier, slower-moving molecules that have heavier carbon isotopes. So, it’s possible to examine a carbon sample’s isotope ratios and determine whether it’s of biological origin. (Not only that, but different carbon-fixing enzymes are better or worse at snatching carbon dioxide from the atmosphere, so with modern carbon samples it’s possible to determine what sort of organism actually did the job—we can even determine how much of you is made from corn-based carbon vs. carbon fixed by wheat and other crops!)
The carbon isotopes reported with these microfossils check out as biological, which is reassuring. There are some arcane ways to make slight isotope imbalances without using biological processes, but the carbon compounds that are made are not consistent with what was found.
The researchers also presented evidence of isotope fractionation with sulfur found in the samples. This is particularly suggestive about what may have occurred so long ago. One place you may think of sulfur in your own biology (especially after the posts from this last week!) is in flatulence—and in a very real way, what is being looked at here are 3.4 billion year old bacteria farts.
The way we get most of our energy is through what are called oxidation-reduction, or “redox” reactions. In these reactions, an electron goes from a high-energy situation to a low-energy situation. Electrons don’t really like to be attached to carbon, so that’s a high-energy situation; electrons love to be attached to oxygen, so that’s a low energy situation. So, we get energy by letting electrons move (accompanied by a proton, to make a hydrogen atom) from carbohydrates to oxygen. To a very rough degree, you can think of these reactions moving hydrogen atoms from carbon to oxygen:
C6H12O6 + O2 --> CO2 + H2O **
There’s plenty of evidence that 3 billion years ago, there was no oxygen in the atmosphere. However, something like this process probably went on, and goes on today in your intestines, where there is no oxygen available. Although electrons love to be on oxygen, sulfur is a pretty good second choice. So, the microbes in your gut can do this reaction:
C6H12O6 + S --> CO2 + H2S
The cells won’t get as much energy, but they’ll get enough to live—and your farts will smell like rotten eggs.
Surrounding the supposed fossils, the researchers found grains of pyrite. If this metabolism (or one very like it, using hydrogen instead of carbohydrate and sulfate instead of sulfur) occurred, the resulting H2S would react with iron in the environment and form pyrite. This reaction can happen without biological intervention, but if it’s done by life as we know it, you would again expect to see an enrichment of heavier isotopes of sulfur—and this is just what was found. The spatial relationship of the supposed fossils and the pyrite grains is also the same as what one sees with modern cells performing this type of metabolism.
So, Brasier and his colleagues present a very good (but I hesitate to say iron-clad) case for having discovered 3.4 billion year old evidence for life. Their geology and taphonomy are sound, and they provide a couple of lines of evidence consistent with life, especially life under the conditions of the young earth. How young was the earth when these maybe-cells maybe lived? There’s geological evidence that the young earth was pummeled by a massive barrage of meteorites. This “late heavy bombardment,” which would have made life impossible by vaporizing the oceans, ended about 3.8 billion years ago. So, as one news report has it, life may have emerged “very soon” after the late heavy bombardment—although I think of 400 million years as being a pretty long time.
This is some impressive work, and it may well be the “oldest evidence for cellular life.” However, as I mentioned, I’m not sure it matters beyond who gets bragging rights. Although it’s old, it doesn’t change anyone’s estimate about when life started. It doesn’t change our views of ancient biochemistry, as it is consistent with what has been predicted. It would be nice to know if the purported cells were Bacteria or Archaea, but that information can’t be recovered. Finally, unambiguous proof of cells that old (or for that matter, if we every find anything on Mars or Europa) is just about impossible.
On the bright side, I suppose it is nice not only to know that you had a great-great grandmother, but also to have a picture of her. It makes for a better lecture to show a slide of these pictures rather than a slide of isotope data.
*David Wacey, Matt R. Kilburn, Martin Saunders, John Cliff and Martin D. Brasier (2011). Microfossils of sulphur-metabolizing cells in 3.4-billion-year-old rocks of Western Australia. Nature Geoscience (Published online 21 August 2011 | DOI: 10.1038.
**Yes, I'm not balancing the equation; I'm just interested in the chemicals, not their amounts. If you're concerned, I encourage you to do the stoichiometry.
Monday, August 22, 2011
Nature vs. Nurture in the gut microbiota III: what's in a name?
The population of microbes in our gut are a personal trait, like height or weight. The precise make-up of that population, its balance of different types of microbes, is significant as it can have major beneficial or harmful effects on our health. Like many other traits, the composition of that population is partly due to nature—our genes create an environment welcoming to certain types of microbes—and nurture, the food we eat.
Given the importance of the composition of the microbial community to our health, it’s not surprising that there is a lot of interest in trying to figure out just how significant are the contributions of nature and nurture. Comparing genetically identical mice with a drastically simplified community of microbes in their gut, researchers found that changes in diet led to predictable changes in community structure. However, comparing genetically distinct strains of laboratory mice, a different group of researchers found that the subtle genetic differences between two inbred lines of mice led to measurable differences in the gut microbial community (see part I).
The roles of nature and nurture can be probed further by comparing the microbial community of different, but related, types of animals. What if two animals are closely related, but have different diets? What if they are only distant relatives, but have similar diets? An extreme example of the latter case is a comparison of the intestinal microbes of the tammar wallaby and the cow (see part II). While the comparison is informative, these two mammals are about as distantly related as possible, so a closer comparison is useful.
In a recent study from Washington University at Saint Louis, a group led by Jeffrey Gordon attempted to compare a family tree of mammals, a family tree of the microbes that lived in their guts, the diets of those mammals, and the genetic abilities of their microbes. Any four-way comparison gets complicated pretty quickly, but there are some clear facts that emerge from the fog of statistics. These facts emphasize some basic biology of microbes, as well as telling us something about how mammals and their microbes co-evolve.
A family tree of the mammals comes out pretty much the same, regardless of whether you use genetic data or anatomical differences:
Looking at this family tree, there is little convergence between relatedness and diet. For instance, elephants, capybaras, gorillas, horses, and rabbits are all “hindgut-fermenting herbivores,” with a specific method of digesting cellulose—and yet, are found on different branches of the tree. Sheep, hyraxes, kangaroos, colobus monkeys, and pigs are all “foregut-fermenting herbivores,” with an anatomically distinct way of digesting plant matter—but again, they are only distant relatives. One can find groups, such as the primates, comprising foregut-fermenters, hindgut-fermenters, carnivores, and omnivores. So, genetics on a deep level does not determine diet.
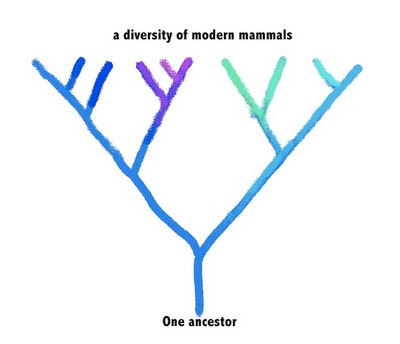
If genetic heritage doesn’t determine diet, does it at least determine something about a mammal’s intestinal microbial community? To approach this question, you can imagine a goodly branch of that mammalian family tree. A diverse bunch of modern mammals evolved from a common ancestor:
We obviously don’t know anything of the DNA sequence of their common ancestor, but we can see the genetic similarities of the modern animals, and work backwards to predict what their ancestor was like, as well as which modern animals are most closely related. If the ancestor had a certain genetic composition—symbolized by the color blue—then its descendants would have evolved variations on that genetic composition—symboized by variations in the hue in the modern beasts.
Now, if genetic heritage determines an animal’s gut microbes, then the genetic character of that community should show a pattern of evolution sort of like the mammalian family tree:
This is a trickier tree than the previous one. Rather than looking at a lineage of organisms, we are looking at the composition of a community of thousands of species. We can make an analogy with the white pages of a phone book: in this tree, we are looking at how the content of a community’s phone book changes over time. (Also, it’s important to remind ourselves that we don’t know what the original community was like—we only have the present one, and we try to work backwards.)
So, what did Gordon’s group find? If mammals and their intestinal microbes co-evolved, then we could examine a diversity of modern mammals and the microbial communities in their poop—which is just what they did. We’d expect related mammals to have related communities. Lining up the tips of the family trees (that is, just currently living animals and the microbes in their poop), we’d see something like this:
There was good reason to expect this result, as similar patterns of co-evolution are seen with insects and their symbiotic bacteria. Co-evolution is common. But here’s what they found:
What to make of this? Obviously, genetic heritage has very little to do with the community structure of an animal’s intestinal microbes. However, Gordon’s group looked at the same data again. This time, instead of looking at mammals by their ancestry, they considered their diet:
Aaaaah, much better! So, it seems like diet—nurture, in our nature/nurture dichotomy—is a much better predictor of a mammal’s microbial community. (This is a much simplified version of the data presented; my apologies to Gordon et al, who studied 33 mammal species and hundreds of microbial species. It’s also useful to note that in several instances they looked at the communities of different individuals of the same species, and found little variation between them.)
Interestingly, despite the differences in community structure between mammals, all mammals have pretty much the same “core” community of microbes. To return to the metaphor of a phone book, any big city in this country will have roughly the same collection of names, it’s just that one city will have a lot more Schmidts and Webers and Mullers and another will have many more Vasquezes and Diazes and Gonzaleses.
Although there was a decent correlation between mammalian diet and community composition in Gordon’s data, his group found an even more significant relationship. The best correlation was not between mammalian diet and community composition. It was between mammalian diet and what genes were present in the microbial community.
Let’s consider that phone book analogy again. If I wanted to find somebody to turn some wheat into flour, I probably wouldn’t turn to the white pages and call up somebody named Muller or Miller. (Heck, I wouldn’t say I’m a good horticulturalist because I’m named Appleman.) Trades are not inherited with family names, and this is true in the microbial world as well. So, if you’re a vegetarian mammal looking for a microbe to turn your b-glucosides into monosaccharides, you can’t assume that you’re looking for Brevibacter—all you care about is whether the organism has the genes for doing the job.
Microbial genomes—the collection of genes a given species has—are incredibly fluid. Genes can disappear from one genome in the space of a few generations, and can establish themselves in a new genome in an equally short time. Genes can travel with relative ease between bacterial species that are barely related to each other. (There are many mechanisms for doing this, and it makes firmly describing a microbial species next to impossible.) So, when a hyrax feeds its intestinal microbes a blend of grass and insects, it is (unwittingly) selecting a specific assemblage of genes, not a specific community of microbes.
These results raise some interesting questions about evolution. The community of microbes that lives in a mammal’s gut—its microbiome—can easily be viewed as an organ like the lungs or kidneys. However, its inheritance and evolution are quite distinct from that of the rest of the animal. As Gordon’s group suggests, it would be fun to compare a lot of different carnivores, and try to extrapolate just when differences in their microbiomes evolved. Do such evolutionary jumps correlate with events such as continental drift or other significant milestones? Are the changes gradual or rapid? Can one community be dislocated by another? Just how fluid are the communities in the gut?
These questions bring us back to the tammar wallaby and its oddly non-methanogenic digestion. Interestingly, in this study, the microbiome of a kangaroo clearly grouped with other foregut-fermenting mammals, such as sheep and gazelles. It was an outlier in that group, but still clearly part of it and not some other group. Well, the tammar wallaby was found to have a small number of methanogens and acetogens in its gut, giving it at least some similarity to the sheep. The big difference in digestion (and flatus) may be traceable to the very small community difference of one organism with one interesting metabolic pathway, a factor that simply can’t be revealed by this sort of analysis. The situation is reminiscent of human societies, which can be hugely affected by a single individual—and knowing the name of that individual won’t tell you anything about how they affect society.
Brian D. Muegge, Justin Kucyznski, Dan Knigts, Jose C. Clemente, Antonio Gonzalez, Luigi Fontana, Bernard Henrissat, Rob Knight, and Jeffrey Gordon (2011). Diet Drives Convergence in Gut Microbiome Functions Across Mammalian Phylogeny and Within Humans. Science 332, 970-974.